eNose analysis for early immunotherapy response monitoring in non-small cell lung cancer
Aim: To determine the predictive value of exhaled breath analysis by eNose for the identification of advanced NSCLC patients with PR to anti-PD-1 therapy with 100% sensitivity after six weeks of treatment.
Take home message: ENose breath analysis can quickly and non-invasively identify NSCLC patients responding to anti-PD-1 therapy, offering better accuracy than current biomarkers. This tool could help tailor treatments early, avoiding unnecessary side effects and improving patient care.
Introduction
Immune checkpoint inhibitors (ICIs) have significantly improved survival in metastatic non-small cell lung cancer (NSCLC). However, not all patients benefit from these treatments, making early identification of responders crucial. Traditional biomarkers like PD-L1 expression are not sufficiently accurate. This study explores using exhaled breath analysis with an electronic nose (eNose) as a non-invasive tool for early monitoring of response to anti-PD-1 therapy in NSCLC patients. The hypothesis is that changes in breath patterns due to metabolic effects of effective therapy can distinguish true responders early in the treatment process.
Methods
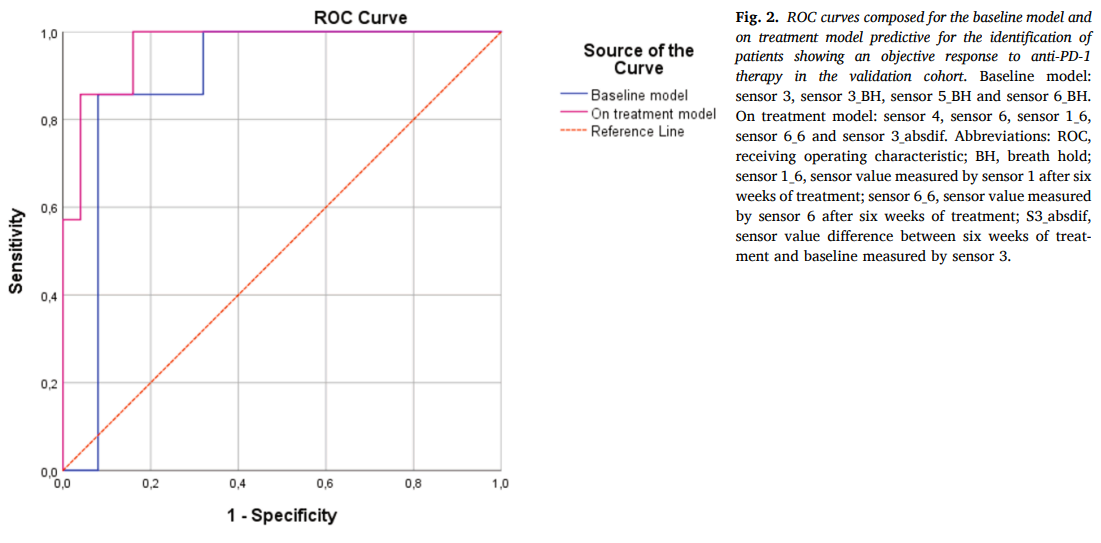
This prospective observational study included 94 NSCLC patients eligible for anti-PD-1 therapy, divided into training (n=62) and validation (n=32) cohorts. Exhaled breath samples were collected at baseline and after six weeks of treatment using the SpiroNose®, which detects volatile organic compounds (VOCs) linked to metabolic changes. Breath profiles were analyzed in real-time on the BreathBase® platform. Two predictive models were developed: one using baseline data alone and another incorporating data from both baseline and six-week follow-ups (the “on-treatment model”). Data were analyzed using Linear Discriminant Analysis (LDA) and validated through Receiver Operating Characteristic (ROC) analysis.
Results
In the training cohort, the on-treatment model achieved a specificity of 73% with a 100% sensitivity and an AUC of 0.95. In the validation cohort, this model’s performance was confirmed with an AUC of 0.97, demonstrating superior predictive capability compared to the baseline model (AUC of 0.89). These results indicate that early changes in exhaled breath profiles are strongly associated with response to therapy, allowing for accurate identification of patients benefiting from anti-PD-1 therapy.