eNose breath prints as a surrogate biomarker for classifying patients with asthma by atopy
Aim: To investigate whether eNoses can classify atopy in pediatric and adult patients with asthma.
Take home message: Electronic nose (eNose) technology can accurately and noninvasively classify asthma patients based on atopic status using breath profiles. This approach offers a promising, rapid tool for asthma phenotyping that could complement traditional methods, facilitating personalized treatment decisions and improving patient care, especially in scenarios where standard testing is impractical.
Introduction
Asthma is a heterogeneous respiratory disease with various underlying mechanisms, including atopy, a common trait related to allergic sensitization. Atopy is typically diagnosed through skin prick tests or IgE measurements, which can be invasive or delayed. This study investigates the use of electronic nose (eNose) technology as a noninvasive tool for detecting atopy in both pediatric and adult asthma patients. eNoses analyze exhaled volatile organic compounds (VOCs) to identify unique breath profiles, potentially providing a faster, point-of-care solution.
Methods
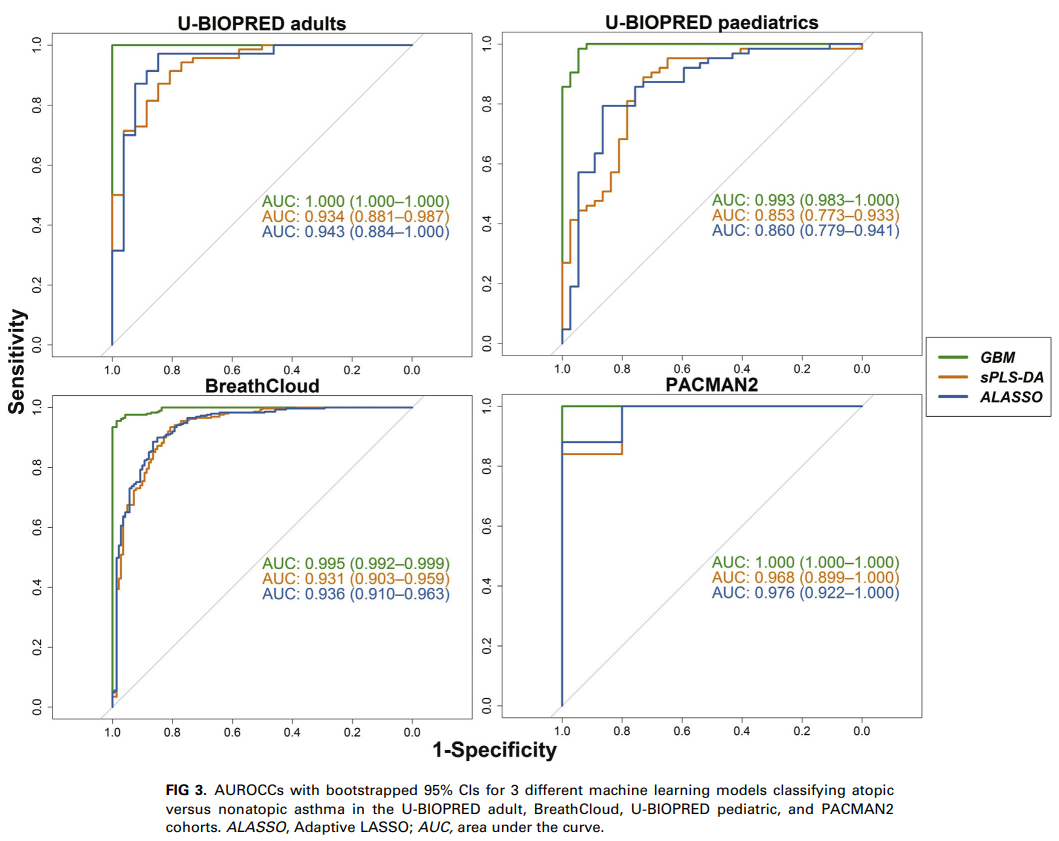
The study included participants from four independent asthma cohorts: BreathCloud, U-BIOPRED (adult and pediatric cohorts), and PACMAN2, involving both children and adults with asthma or wheezing (total n = 655). Atopy was defined as a positive result on a skin prick test or specific IgE measurement. Exhaled breath profiles were collected using offline eNose technology or the real-time SpiroNose® (in combination with the BreathBase® platform). Three machine learning models (sPLS-DA, adaptive LASSO, and gradient boosting machine) were applied to classify atopic and nonatopic patients based on their breath profiles. Additionally, an unsupervised Bayesian network analysis was performed to explore potential relationships between eNose signals and patient characteristics.
Results
The machine learning models demonstrated strong discriminatory ability, with areas under the receiver operating characteristic curve (AUROCC) of at least 0.84 in training sets and 0.72 in validation sets. In the BreathCloud cohort, real-time data collection using SpiroNose achieved an AUROCC of at least 0.91. The models effectively differentiated between atopic and nonatopic participants, and this was consistent across different age groups and independent cohorts. The unsupervised analysis indicated that breath profiles predicting atopy were not confounded by other patient characteristics.